
Marks of a chronic lung disease - IMAGES FROM CLINICAL PRACTICE
Marks of a chronic lung disease - IMAGES FROM CLINICAL PRACTICE
Victor Botnaru1, Irina Voloșciuc1*, Diana Calaraș1, Oxana Munteanu1
1Discipline of pneumology and allergology, Department of internal medicine, Nicolae Testemitanu State University of Medicine and Pharmacy, Chisinau, Republic of Moldova.
Coresponding author:
Irina Volosciuc, assistant professor
Discipline of pneumology and alergology, Department of internal medicine
Nicolae Testemitanu State University of Medicine and Pharmacy
165, Stefan cel Mare si Sfant ave., Chișinau, Republic of Moldova, MD-2004
e-mail: irina.volosciuc@usmf.md
Short title: Marks of a chronic lung disease
Questions:
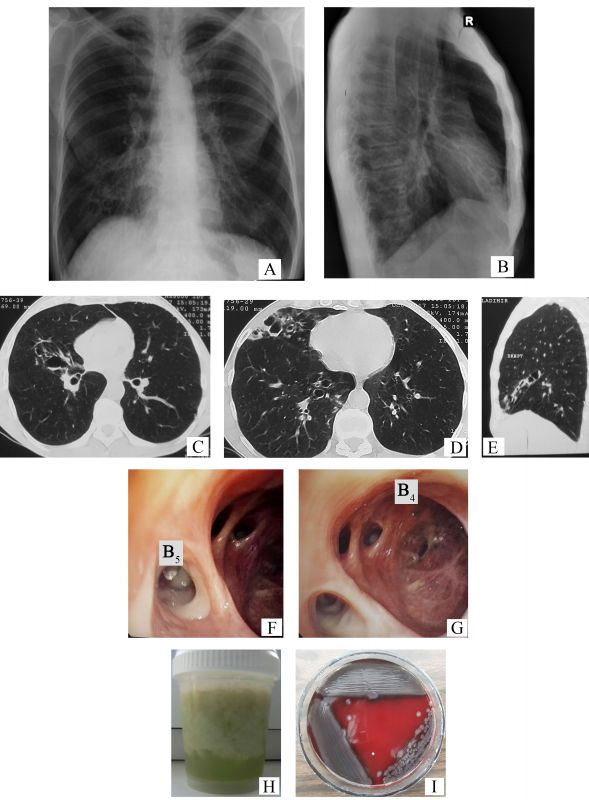
- Describe the chest x-ray changes identified in Figures 1 A and 1 B.
- Name the imaging lesions seen additionally on computer tomography (Figures 1 C-E).
- Describe the endoscopic aspect of the bronchi (Figures 1 F-G) that characterizes the imaging lesions identified on computer tomography.
- Which bacteria is isolated from the sputum culture (another patient), considering the macroscopic aspect of the sputum (Figure 1 H) and the morphology of bacterial colonies (Figure 1 I), on the blood agar medium?
Answers:
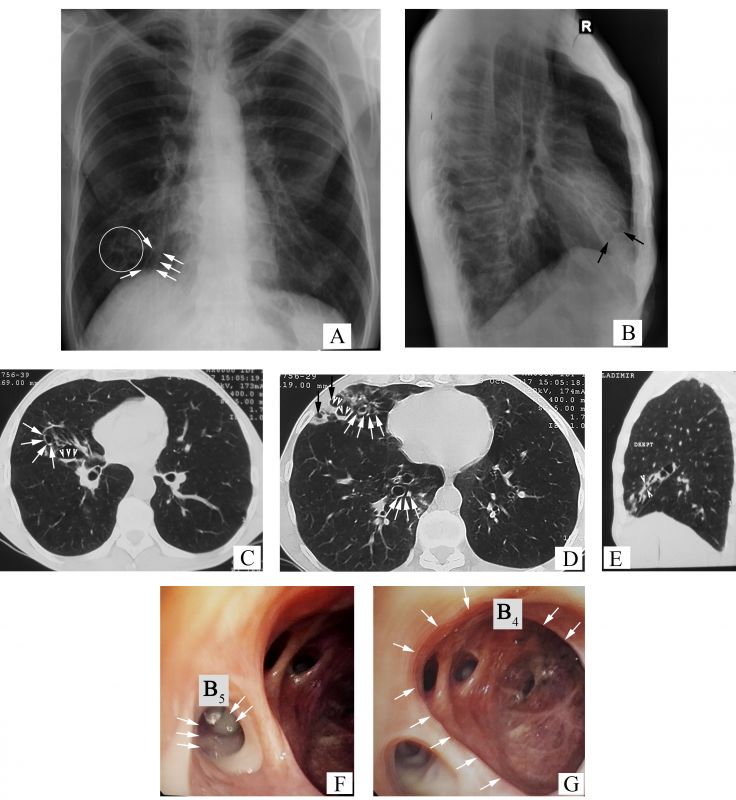
- The chest x-ray images present the tram-track sign – parallel linear densities, that correspond to thickened bronchial walls (arrows, Figure 2 A), multiple ring shadows arranged in ”rosette Ameuille” pattern (circle, Figure 2 A) – considered a characteristic sign of bronchiectasis. Some of them with air-fluid levels (arrows, Figure 2 B), localized in the right middle lobe (RML). Also, traction changes of the lung parenchyma can be seen (apical displacement of the right dome of diaphragm, cardiac shadow on the median line) in the RML (the horizontal fissure close to the oblique one), together with hyperinflation syndrome (hyperlucent lungs, horizontalization of ribs and enlarged intercostal space, drop-shaped heart, barrel chest with increased anteroposterior diameter of the chest with increased retrosternal space).
- On the chest computed tomography (CT), there can be seen the following signs of bronchiectasis: the signet-ring sign – internal bronchial diameter greater than the diameter of the accompanying pulmonary artery (white arrows, Figures 2 C-D), varicose bronchiectasis and cystic bronchiectasis, some of them with endobronchial secretions – air-fluid levels (arrowheads, Figure 2 C and Figure 2 D respectively), the ”finger-in-glove” sign (arrowheads, Figure 2 E) and dilated airways at periphery, close to the subpleural space (normally at this level they cannot be seen ) (black arrows, Figure 2 D).
- Diffuse hyperemia of segmental bronchial mucosa from the RML. Purulent bronchial secretions (arrows, Figure 2 F) in the lumen of the medial segmental bronchi (B5). The orifice and lumen of the lateral segmental bronchi (B4) are dilated (arrows, Figure 2 G), the bronchial mucosa is atrophic with scarring, subsegmental bronchial orifices are narrowed and deformed.
- Pseudomonas aeruginosa. The greenish tint of the sputum expresses a high degree of the purulence (especially stagnant sputum), and it is determined by an increased level of myeloperoxidase (verdoperoxidase) released from neutrophils. A green tint of bronchial secretions can be seen in infections with different potentially pathogenic microorganisms (PPM), like Pseudomonas aeruginosa, Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, and Klebsiella pneumoniae, but a deep green color with blue-green tint is characteristic for the pyocyanic bacillus (”the blue pus bacillus”) due to production of specific pigments. Besides bronchiectasis, it is highly prevalent in infections of burnt patients, in severe trauma, in sepsis, in eye lesions (keratitis, endophthalmitis), otitis media, and in respiratory infections of immunosuppressed patients. On the blood agar medium, the colonies of Pseudomonas aeruginosa are hemolytic and have a metallic sheen [1].
CLINICAL CASE
A 52-year-old man with a 35 pack-year history of tobacco use, was referred to a pulmonologist in September 2017, for a persistent cough with purulent sputum (50 ml/24 hours), dyspnea on moderate exertion, and wheezing. His history was inconsistent for treated tuberculosis, but in 1985 he had right-sided pneumonia, after which chronic cough persisted with or without expectorations of different amounts. Considering the above-mentioned symptoms the family physician diagnosed him with chronic bronchitis. During the last 5 years, his respiratory symptoms worsened (increased bronchorrhea and dyspnea) 2-3 times per year. On physical examination, the patient was underweight (BMI 18 kg/m2), no leg edema, with bilateral reduction of breath sounds, and normal oxygen saturation (SpO2 was 96% while breathing room air). Spirometry parameters were within normal limits (FEV1 99%, FVC 93%, FEV1/FVC 86% of the predicted). The presence of bronchiectasis in the RML was suspected on the chest x-ray and confirmed by CT. Pseudomonas aeruginosa was isolated from sputum culture.
DISCUSSION
Bronchiectasis is a chronic respiratory disorder of the airways, anatomically defined by abnormal and irreversible dilatation of the bronchi, clinically characterized by cough, expectoration and recurrent respiratory infections [2]. In adults, the main symptom is chronic cough with mucopurulent sputum (up to 80% of cases). As the severity of the disease increases, other signs and symptoms could associate (such as dyspnea, hemoptysis, weight loss and fatigue) [3]. Bronchiectasis can be identified in individuals of any age, however, their prevalence increases with age. According to the studies from Germany (2015), prevalence of bronchiectasis in general population is 67 cases per 100,000 population, with vertiginous growth in those over 75 years old (exceeding 200 cases per 100,000 population) [4]. Bronchiectasis can be the final common pathway of several infectious (pneumonia, tuberculosis), allergic (bronchial asthma), genetic (cystic fibrosis) and degenerative disorders [5].
Bronchial dilatation could be suspected on a chest x-ray, historically they were confirmed on bronchography. Nowadays, bronchography has been replaced by CT, an investigation method that is safe, an easily tolerated, that gives much more information [2].
CT signs of bronchiectasis are bronchoarterial ratio (BAR) >1, parallel linear opacities (the ”tram-track” sign), lack of normal bronchial tapering, visibility of airways at the periphery of the lungs [5].
A ratio of the internal bronchial diameter to the diameter of the accompanying pulmonary artery above 1 is considered pathognomonic and defines the ”signet-ring” sign. Accuracy of this sign is limited, being influenced and by other factors, such as elevated altitude (due to hypoxic bronchodilatation and vasoconstriction an increase of BAR and decrease of the bronchial wall thickening), CT section plan (a right CT section plan has to be orientated perpendicularly on the airways), age of the patient (BAR increases with age) [6-9]. Also, there is evidence that in patients with chronic obstructive pulmonary disease, the BAR is increased rather due to vasoconstriction than due to enlarged bronchial diameter [10]. Given the possibility of false-positive diagnosis of bronchiectasis according to the BAR, it is recommended that the diagnosis of bronchiectasis should not rely on radiology only, but also airway neutrophilia should be considered [5]. Lack of bronchial tapering is another criteria for bronchiectasis on CT (bronchial diameter should remain unchanged for at least 2 cm distal to a branching point – Figure 4 A). Normal bronchi usually are not visualized within 1 cm of the costal pleura. Once they become visible, an abnormal dilatation of the bronchi is ascertained (black arrows Figure 4 B) [6].
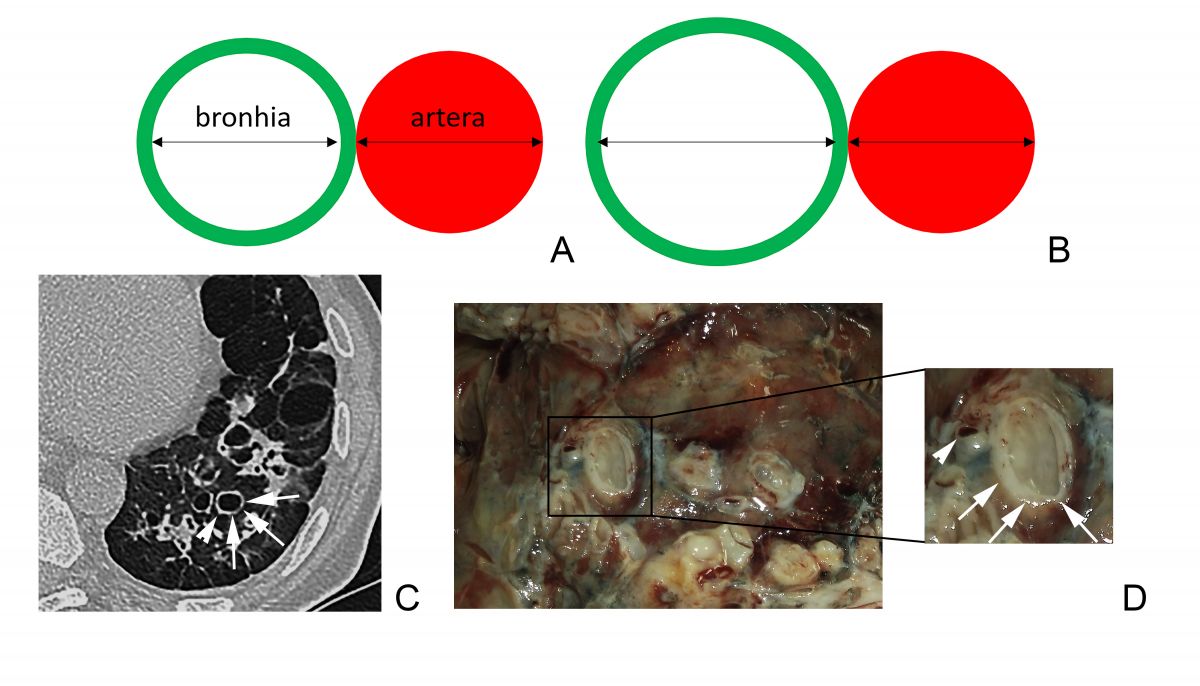
Fig. 3 Broncho-arterial ratio. A. BAR = 1, normal value. B. BAR >1 characteristic for bronchiectasis. C. Thoracic CT plane with the ”signet-ring” sign – dilated bronchi (arrows) next to an artery with a normal diameter (arrowhead). D. (Contribution of Dr. Haidarlâ I.) Morphologic aspect dilated bronchi (arrows) next to an artery with a normal diameter (arrowhead).
Ancillary signs of bronchiectasis on CT include bronchial wall thickening (white arrows, Figure 4 B), mucoid impaction of dilated large-caliber bronchi – the ”finger-in-glove” sign (arrows, Figure 4 C) and of the small bronchi – the ”tree-in-bud” sign (white arrowheads, Figure 4 B), air-trapping (black arrowhead, Figure 4 B) [6].
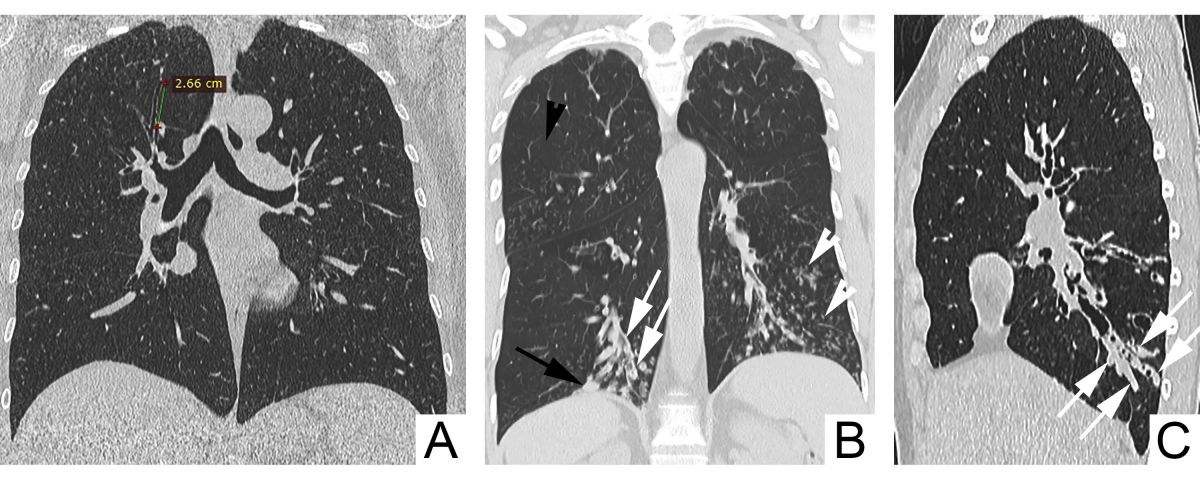
Fig. 4 Signs of bronchiectasis on CT. A. Nontapering bronchi with a constant diameter over >2 cm. B. Visible bronchi within 1 cm of the costal pleura (black arrow), bronchial wall thickening (white arrows), mucoid impaction of small caliber bronchi – the ”tree-in-bud” sign (white arrowheads), air-trapping (black arrowhead). C. Mucoid impaction of dilated large-caliber bronchi – the ”finger-in-glove” sign (arrows).
The different morphological types of bronchiectasis corresponding to the bronchographic classification of Reid, show different imaging features, so that, in cylindrical bronchiectasis uniform dilatation of the bronchi with nontapering walls is identified (white arrowheads, Figure 5), in varicose ones – there are dilated bronchi with a beaded appearance (white arrows, Figure 5), but in cystic bronchiectasis a ”cyst-like” gross bronchial dilatation is visualized (black arrows, Figure 5) [11].
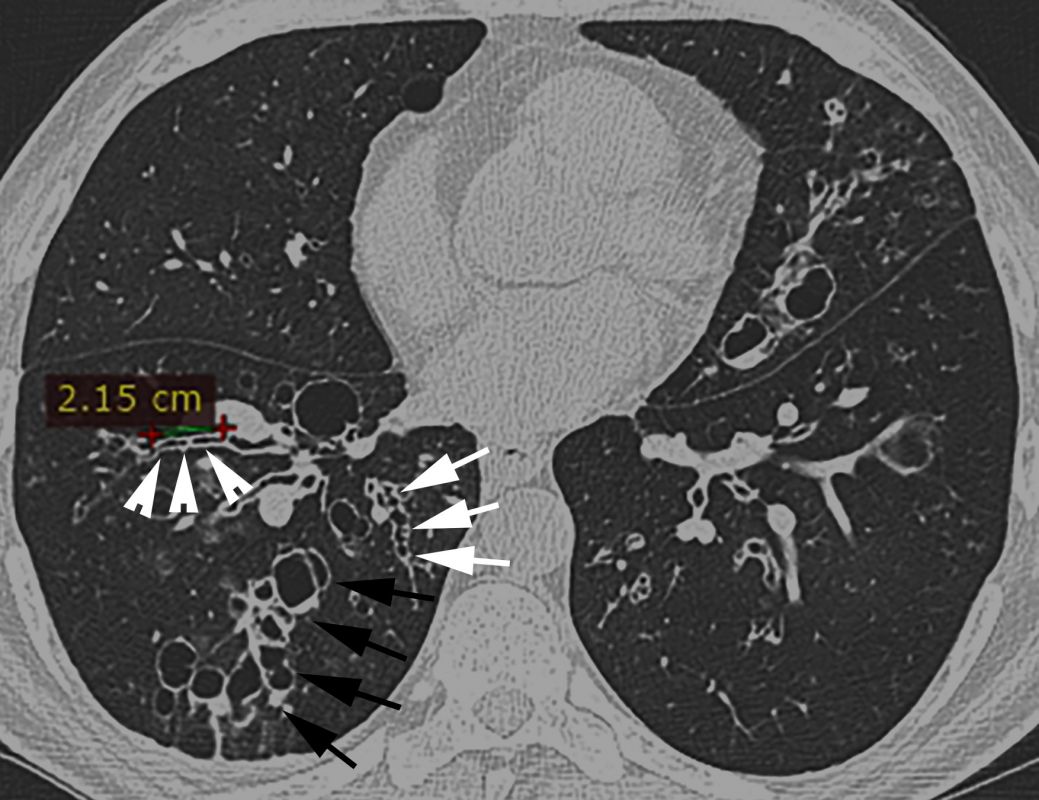
Fig. 5 Morphological types of bronchiectasis: cylindrical (white arrowheads), varicose (white arrows), cystic (black arrows).
The clinical and radiological evolution of bronchiectasis depends on the isolated pathogen from sputum or bronchoalveolar lavage. At least one PPM (Pseudomonas aeruginosa, Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, Staphylococcus aureus) is identified in sputum culture in 80% of cases, Pseudomonas aeruginosa being the most frequent [5, 6].
Pseudomonas aeruginosa, commonly named pyocyanic bacillus (”the blue pus bacillus”, due to the capacity to color the pus in green-blue), is a gram negative, mobile, oxidase positive and asporogenous bacteria. It is ubiquitous, being encountered in soil, water, plants, decaying organic matter, but also in the hospital (sewerage systems, flower vases, artificial ventilation equipment, some disinfectants), where it causes nosocomial infections, that due to multidrug resistance to antibiotics are difficult to treat. In humans, it may be a part of the normal intestinal or skin flora. Although it has a wide variety of virulence factors (presence of the pili, production of proteases with histotoxic effects, hemolysins and exotoxins synthesis and the presence of an endotoxin), Pseudomonas aeruginosa is potentially pathogenic [12]. In order to induce the disease, a collapse of the host protection mechanisms is needed, by damaging the integrity of the mechanical barriers (skin, mucous membrane) or by the presence of immune deficiencies (neutropenia, immunosuppression) [13].
Pyocyanic bacillus identification is simple due to its cultural character. On simple agar medium or broth, it is turning the medium to green due to pyocyanin secretion (blue phenazine, non-fluorescent, water soluble pigment), that is combining with pyoverdine (yellow-green, fluorescent pigment) [12]. On the blood agar medium the colonies appear shiny, with a metallic appearance, hemolytic (Figure 1 I) and release a characteristic ”grape-like” odor [14].
In patients with bronchiectasis, pyocyanic bacillus isolation is associated with the presence of severe bronchiectasis (characterized predominantly by a cystic morphological pattern and a polysegmental distribution), with a more rapid progression of pulmonary lesions, with an increase of mortality and hospital admission rate, and with a much more reduced quality of life [15-17].
CONCLUSIONS
Bronchiectasis should be suspected in a patient with chronic respiratory symptoms and important bronchorrhea. Computer tomography is the method of choice for confirmation in case of the presence of bronchiectasis characteristic imaging signs on the chest x-ray (ring opacities, the ”tram-track” sign). The presence of an MPP may be suggested by the macroscopic aspect of the sputum, such as Pseudomonas aeruginosa, which identification is a marker of an unfavorable prognosis and requires a particular approach.
Declaration of conflicting interests
Nothing to declare.
REFERENCES
- Miravitlles M. et al. Sputum colour and bacteria in chronic bronchitis exacerbations: a pooled analysis. European Respiratory Journal, 2012; 39 (6): 1354-1360.
- Botnaru V., Munteanu O., Balica I., Calaraș D. Bronșiectaziile la adult. Protocol Clinic Național. Republica Moldova, 2017. 275: p. 27-31.
- Dimakou K., Triantafillidou C., Toumbis M., Tsikritsaki K., Malagari K., Bakakos P. Non CF-bronchiectasis: aetiologic approach, clinical, radiological, microbiological and functional profile in 277 patients. Respiratory Medicine, 2016; 116: 1-7.
- Ringshausen F. et al. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur. Respir. J., 2015; 46 (6): 1805-7.
- Chalmers J., Chang A., Chotirmall S., Dhar R., McShane P.. Bronchiectasis. Nature Reviews Disease Primers, 2018; 4 (1): 45.
- Floto R., Haworth C. Bronchiectasis. European Respiratory Society Monograph, 2011; 52: 32-43.
- Matsuoka S. et al. Bronchoarterial ratio and bronchial wall thickness on high-resolution CT in asymptomatic subjects: correlation with age and smoking. AJR Am. J. Roentgenol., 2003; 180 (2): 513-8.
- Matsuoka S., Uchiyama K. et al. Bronchoarterial ratio and bronchial wall thickness on high-resolution CT in asymptomatic subjects: correlation with age and smoking. American Journal of Roentgenology, 2003; 180 (2): 513-518.
- Joung K., Müller N. et al. Bronchoarterial ratio on thin section CT: comparison between high altitude and sea level. Journal of Computer Assisted Tomography,1997; 21 (2): 306-311.
- Diaz A. et al. Bronchoarterial ratio in never-smokers adults: implications for bronchial dilation definition. Respirology, 2017; 22 (1): 108-113.
- Reid L. Reduction in bronchial subdivisions in bronchiectasis. Thorax, 1950; 5: 233-247.
- Licker M. ş.a. Curs de microbiologie specială, îndreptar de lucrări practice. Ed. „Victor Babeş”, Timişoara, 2019.
- Bassetti M., Vena A., Croxatto A., Righi E., Guery B. How to manage Pseudomonas aeruginosa infections. Drugs in Context, 2018; 7: 212-527.
- Lederberg J. et al. Pseudomonas. Encyclopedia of Microbiology. Second Edition. Volume 3. San Diego, 2000. p. 876-891.
- Finch S., McDonnell M., Abo-Leyah H. et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann. Am. Thorac. Soc., 2015; 12: 1602-1611.
- Lynch et al. Correlation of CT findings with clinical evaluations in 261 patients with symptomatic bronchiectasis. American Journal of Roentgenology, 1999; 173 (1): 53-58.
- Park J. et al. Factors associated with radiologic progression of non-cystic fibrosis bronchiectasis during long-term follow-up. Respirology, 2016; 21 (6): 1049-54.
